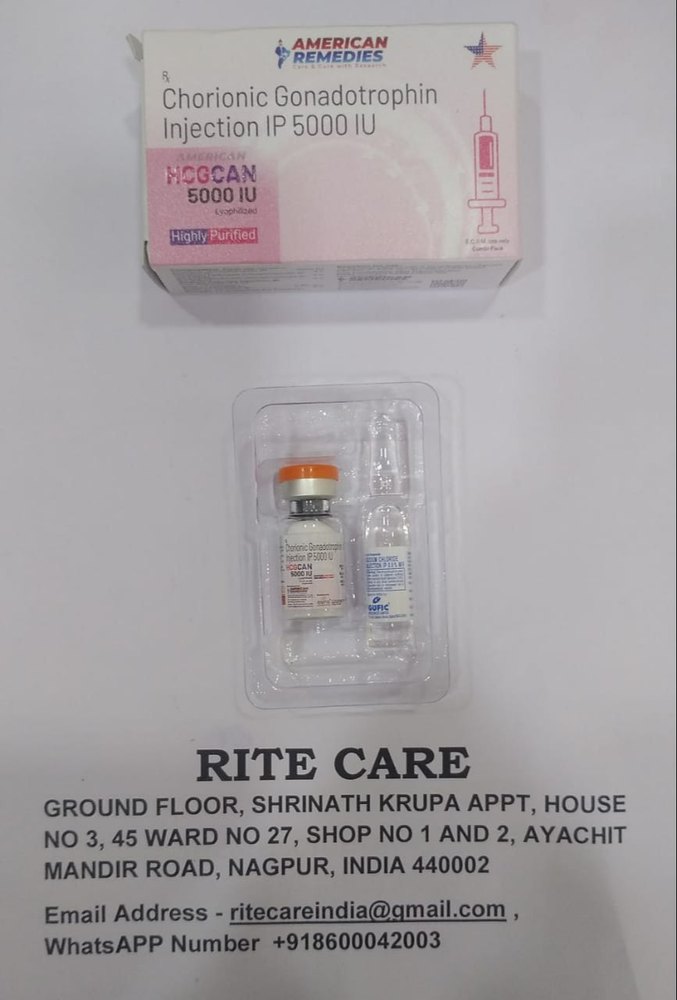
HCG 5000 IU Injection contains Human Chorionic Gonadotropin, a hormone used for a variety of medical and therapeutic applications. HCG mimics luteinizing hormone (LH) in the body, stimulating the testes to produce testosterone in males, and inducing ovulation in females.
This product comes in lyophilized powder form and must be reconstituted with sterile or bacteriostatic water before use.
Primary Medical Uses:
-
Treatment of male hypogonadism and infertility
-
Induction of ovulation in women with fertility issues
-
Part of assisted reproductive treatments (ART)
-
Used in testosterone replacement therapy (TRT) to maintain fertility and testicular function
-
Occasionally used in weight loss protocols (off-label, not medically recommended without oversight)
Key Features:
-
High-potency 5000 IU dose per vial
-
Supports natural testosterone production
-
Preserves fertility during TRT cycles
-
Injectable for rapid systemic absorption
-
Can be used in medically supervised hormone protocols
Administration:
-
Reconstitution Required: Mix with sterile/bacteriostatic water
-
Injection Site: Subcutaneous (abdomen/thigh) or intramuscular, based on physician guidance
-
Typical Dosage: Varies by condition; commonly 500–2000 IU 2–3 times weekly for men in TRT or fertility protocols
Storage Instructions:
-
Before Mixing: Store in a cool, dry place (preferably refrigerated at 2°C to 8°C)
-
After Reconstitution: Keep refrigerated and use within 30 days (or as instructed)
-
Protect from heat, moisture, and light
Precautions & Side Effects:
-
Should only be used under medical supervision
-
Not for use in individuals with hormone-sensitive cancers
-
Possible side effects: acne, mood swings, water retention, gynecomastia, injection site reactions
-
In women: risk of ovarian hyperstimulation syndrome (OHSS)